Why One of Lung Cancer’s Best Drugs Eventually Stops Working- and How Nuvectis Pharma Is Trying to Fix That
In modern oncology, progress is often defined less by discovering new drugs than by understanding why good drugs stop working.
Few therapies illustrate that challenge more clearly than AstraZeneca’s Tagrisso (osimertinib), the backbone treatment for patients with EGFR-mutated non-small cell lung cancer (NSCLC). Tagrisso generates more than $6 billion in annual sales and delivers meaningful responses for many patients. Yet despite its success, most patients eventually relapse as tumors adapt and develop resistance.
That biological wall—acquired resistance—has become one of the most stubborn problems in lung cancer care. And it is precisely that bottleneck that a small, under-the-radar biotech called Nuvectis Pharma (NASDAQ: NVCT) is now attempting to address.
A Sophisticated Strategy by
The company’s lead asset, NXP900, is now advancing through a Phase 1b clinical program that includes both biomarker-selected single-agent cohorts and combination cohorts in resistant lung cancer. The osimertinib combination represents an important part of the strategy—but it is not the entire thesis.
That distinction matters. Replacing a well-established standard-of-care drug is extraordinarily difficult. Extending its benefit in patients who have already exhausted it is both clinically meaningful and strategically pragmatic. At the same time, maintaining a standalone development path allows Nuvectis to test whether SRC-pathway inhibition can drive antitumor activity on its own in selected populations.
Why Resistance Happens
Scientific research over the past several years has shown that resistance to EGFR inhibitors is often not random. In many tumors, resistance emerges through activation of alternative signaling routes involving SRC family kinases, including SRC and YES1. These pathways can bypass EGFR blockade, allowing cancer cells to survive even in the continued presence of osimertinib.
Nuvectis’ core hypothesis is straightforward but ambitious: if that escape route can be shut down, the benefit of EGFR inhibition may be prolonged—or, in some cases, restored.
What Makes NXP900 Different
SRC has been targeted before, but with limited success. Prior drugs often struggled to adequately suppress SRC signaling or ran into tolerability issues that limited their usefulness in solid tumors.
NXP900 was designed with those challenges in mind. Rather than focusing solely on catalytic inhibition, the drug is engineered to inhibit both the catalytic activity of SRC family kinases and their non-catalytic scaffolding functions, a feature that has historically been difficult to achieve.
Clinically, the groundwork for this approach has already been laid. Nuvectis has completed a Phase 1a dose-escalation study of NXP900 in patients with advanced solid tumors, as well as a dedicated drug–drug interaction study in healthy volunteers. These studies were designed to establish dosing, safety, and pharmacologic behavior—not to prove efficacy—and to enable subsequent combination testing.
That foundation now supports two parallel Phase 1b efforts: single-agent cohorts selected by genomic alterations thought to confer SRC-pathway dependence, and combination cohorts in patients with acquired resistance to targeted therapies.
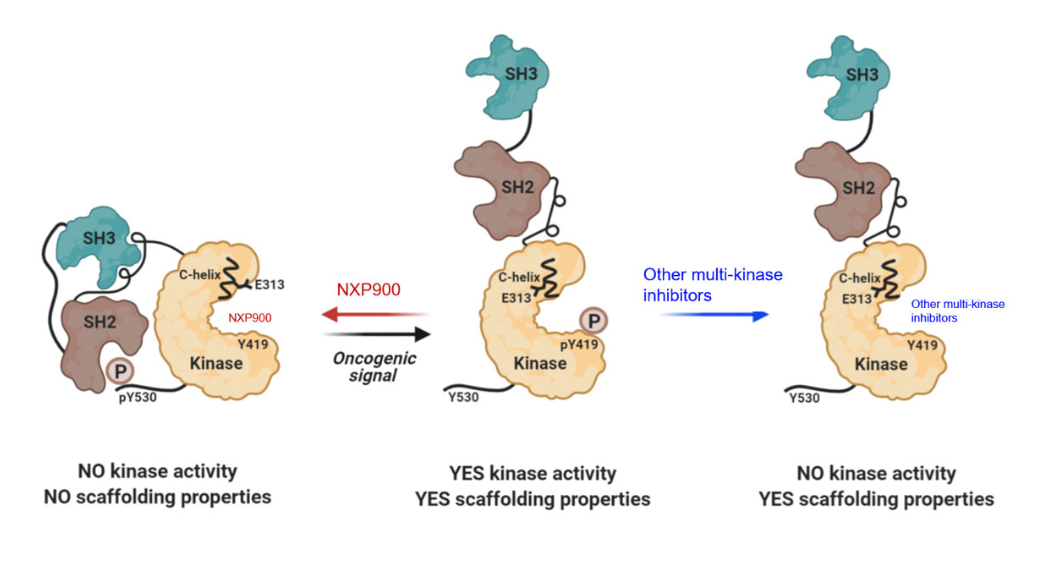
Testing the Hypothesis Where It Matters Most
In December, Nuvectis initiated a Phase 1b study evaluating NXP900 in combination with osimertinib in patients with EGFR-mutated NSCLC whose cancers initially responded to Tagrisso but later progressed.
The study is not positioned as a definitive efficacy trial. Its purpose is to evaluate safety, tolerability, and early signals of activity when NXP900 is added in a resistance setting, an important distinction in a patient population with few remaining options.
Preclinical work provided the rationale for this step. In laboratory models, tumors treated with EGFR inhibitors alone initially responded but later rebounded as SRC-mediated resistance pathways activated. When NXP900 was added, tumor suppression was enhanced and more durable, supporting the biological logic behind the combination.
Institutional Signals Still Matter
One of the more telling aspects of the program did not come from a headline announcement, but from the study leadership itself.
The NXP900–osimertinib combination trial is being led by Dr. Zofia Piotrowska at Massachusetts General Hospital, a leading authority in EGFR-mutant lung cancer and a key investigator in AstraZeneca’s landmark FLAURA program. At institutions like MGH, early-stage combination studies typically undergo rigorous internal review, and senior clinicians are selective about the programs they choose to lead.
For a micro-cap company, securing that level of clinical engagement suggests the study’s rationale and design have met a high bar.
Notably, Nuvectis was founded by a management team with a rare track record of taking drugs all the way across the finish line. The company is led by Ron Bentsur, who has been involved in the development and approval of multiple oncology drugs through his prior work at Keryx Biopharmaceuticals and UroGen Pharma, where he served as CEO. He is joined by Enrique Poradosu, PhD, and Shay Shemesh, whose combined experience includes three approved drugs across four indications in the U.S. and Europe, as well as multiple strategic and commercial partnerships.
Why Watch Nuvectis Now?
Nuvectis remains a company most have not heard of, operating in the shadow of one of oncology’s most valuable franchises. That asymmetry is precisely what makes the story worth watching.
If resistance to EGFR inhibitors is one of the most persistent challenges in lung cancer—and if SRC-mediated bypass signaling proves clinically actionable—then the outcome of this program could matter beyond a single drug or company.
From a business standpoint, the company is particularly well funded having reported sufficient cash to support operations into 2027, giving it time to advance both its monotherapy and combination programs without immediate financing pressure. Management has also drawn attention for open-market share purchases, which many have interpreted as a sign of their strong conviction in the program.
There are no guarantees in early-stage biotech. Many programs fail. But Nuvectis has now crossed an important threshold: it has moved from hypothesis to execution, from preclinical rationale to carefully designed clinical tests in populations with clear unmet need.
Sometimes, the most important developments don’t come from the biggest companies - but from the smallest ones taking aim at the biggest problems.
Read this next >> Alpha Tau’s Radiation Therapy Shows it Could Treat Pancreatic Cancer While Preserving the Immune System
Latest News Highlights from Nuvectis:
Nuvectis Pharma Appoints Biotech Executive Juan Sanchez, MD, to the Board of Directors
Important Disclaimers and Disclosures: The author, Wall Street Wire, is a content and media technology platform that connects the market with under-the-radar companies. The platform operates a network of industry-focused media channels spanning finance, biopharma, cyber, AI, and additional sectors, delivering insights on both broader market developments and emerging or overlooked companies. The content above is a form of paid promotional content and advertising. Wall Street Wire has received cash compensation from Nuvectis Pharma Inc for promotional media services which are provided on an ongoing basis. This content is for informational purposes only and does not constitute financial or investment advice. Wall Street Wire is not a broker-dealer or investment adviser. Full compensation details, information about the operator of Wall Street Wire, and the complete set of disclaimers and disclosures applicable to this content are available at: http://wallstwire.ai/disclosures. Market size figures or other estimates referenced in this article are quoted from publicly available sources; we do not independently verify or endorse them, and additional figures or estimates may exist. This article should not be considered an official communication of the issuer.





