This Tiny Biotech Stock Could Unlock a New Approach to Pancreatic Cancer Treatment (NASDAQ: SLXN)
COVID-19 vaccines did more than help end a global pandemic. They also introduced the public to a once-fringe idea: RNA itself can be medicine. Silexion Therapeutics is using RNAi to advance a next generation potential therapy for once of the toughest cancers | NASDAQ: SLXN

COVID-19 vaccines did more than help end a global pandemic. They also introduced the public to a once-fringe idea: RNA itself can be medicine.
Those vaccines relied on messenger RNA (mRNA) to instruct cells to produce a harmless protein that trains the immune system. But mRNA is only one way RNA can be used therapeutically. A related approach, called RNA interference (RNAi), works in the opposite direction - rather than telling cells to make something, it is designed to silence specific genes altogether.
Now, a tiny clinical-stage biotech, Silexion Therapeutics (NASDAQ: SLXN), is betting that gene-silencing technology can help address one of cancer’s most stubborn targets and potentially reshape how pancreatic cancer is treated.
KRAS: From “Undruggable” to Partially Solved
At the center of this story is KRAS, a gene that plays a critical role in cell growth. When KRAS is mutated, it can drive uncontrolled cancer growth. These mutations are present in roughly 90% of pancreatic cancer cases, making KRAS one of the most important - and most difficult - targets in oncology.
For decades, KRAS was widely considered “undruggable.” That perception changed in 2021, when the FDA approved sotorasib for lung cancer patients with a specific KRAS mutation known as G12C. It was a landmark moment, proving that KRAS could, in fact, be targeted with drugs.
The catch is that G12C is rare in pancreatic cancer, accounting for roughly ~1% (generally reported as under 2%) of cases. The most common pancreatic cancer mutations are G12D and G12V, which together represent a large majority of patients - and which current FDA-approved KRAS drugs are not designed to address.
As a result, despite major progress, most pancreatic cancer patients still lack an effective KRAS-targeted treatment. With a five-year survival rate of just 13%, the unmet need remains profound.
A Different RNA Approach
Silexion is pursuing a fundamentally different strategy.
Instead of trying to block the KRAS protein after it has already been made, the company’s lead candidate, SIL204, uses RNA interference to silence the mutated KRAS gene itself. A useful analogy is turning off a faucet rather than trying to mop up water after it spills.
RNA-based therapies have historically struggled with delivery, particularly in solid tumors. SIL204 addresses this challenge through a localized approach. The therapy is injected directly into the pancreatic tumor using a routine endoscopic ultrasound procedure and is packaged in biodegradable microparticles that slowly release the RNA over approximately three months. The goal is to maintain sustained activity at the tumor site while minimizing systemic exposure.
What the Early Data Shows
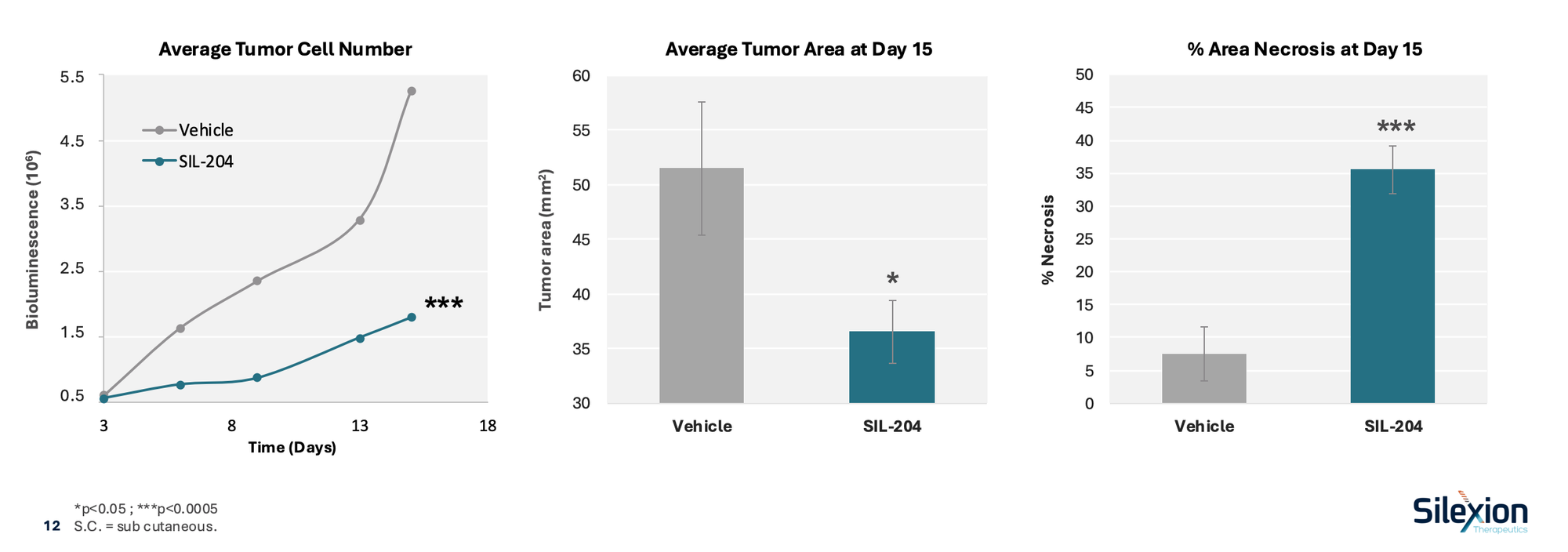
In preclinical laboratory testing, SIL204 demonstrated strong inhibition of cancer cell growth across multiple KRAS mutations, including those most common in pancreatic cancer. In certain human cancer cell models, inhibition reached as high as 99.7%. The company has also reported completion of two-species toxicology studies showing no systemic organ toxicity.
These results are preclinical, and there is no guarantee they will translate into clinical benefit in humans. Still, they help explain why Silexion’s approach stands out: unlike mutation-specific KRAS inhibitors, SIL204 is designed to address multiple KRAS variants rather than just one. While the upcoming human trial focuses on locally advanced pancreatic cancer, this alone represents a multibillion-dollar indication, with broader potential beyond that setting.
Built on Prior Clinical Experience
Silexion is not new to pancreatic cancer. The company previously advanced an earlier version of its localized RNA technology, known as LODER, through a Phase 2 clinical trial in locally advanced pancreatic cancer. In company-reported results, that study showed a 9.3-month improvement in overall survival compared to chemotherapy alone, along with a 56% objective response rate.
SIL204 represents a next-generation formulation built on that experience. While earlier results do not predict future outcomes, they demonstrate that the team has already navigated the clinical and procedural complexities of treating pancreatic tumors directly - a meaningful credibility point for a small biotech.
What Investors Are Watching Next
In late 2025, Silexion submitted a Phase 2/3 clinical trial application to Israel’s Ministry of Health and reported positive feedback from Germany’s regulatory authority on its trial design. Additional regulatory submissions in Europe are planned for early 2026, with a U.S. IND anticipated later in the year.
The planned trial is designed to directly treat the primary pancreatic tumor, with additional evaluation of approaches intended for more advanced disease. The company has also established a manufacturing collaboration with Catalent and, according to its January 2026 CEO letter to shareholders, raised over $18 million in aggregate financing during 2025 - practical considerations that matter for a microcap preparing for multi-site clinical trials.
A Wall Street analyst from Litchfield Hills Research initiated coverage in December 2025 with a Buy rating and a $6 price target for the next 12 months, compared to the current share price in the low-$2 range.
The Bottom Line
Silexion remains an early-stage biotech, and success will ultimately depend on clinical results. That risk is unavoidable.
What makes the current moment notable, however, is where the company sits in its development arc. After years of work building and validating its localized RNA platform, Silexion is approaching a transition point: regulatory feedback, trial initiation, and early clinical execution are now moving from future concepts to near-term events.
For investors, these transition periods are often when perception begins to shift - from “interesting science” to “clinical story with real milestones.” With a defined regulatory path, manufacturing in place, and a treatment strategy that targets a core driver of pancreatic cancer rather than a narrow subset of patients, Silexion is entering a phase where comparable companies are often valued at substantially higher levels, potentially creating a unique opportunity to gain exposure to a company aiming to change how one of the deadliest cancers is treated.
Recent News Highlights From Silexion Therapeutics
Important Disclaimers and Disclosures: The author, Wall Street Wire, is a content and media technology platform that connects the market with under-the-radar companies. The platform operates a network of industry-focused media channels spanning finance, biopharma, cyber, AI, and additional sectors, delivering insights on both broader market developments and emerging or overlooked companies. The content above is a form of paid promotional content and advertising. Wall Street Wire has receives cash compensation from Silexion Therapeutics Corp for promotional media services which are provided on an ongoing basis. This content is for informational purposes only and does not constitute financial or investment advice. Wall Street Wire is not a broker-dealer or investment adviser. Full compensation details, information about the operator of Wall Street Wire, and the complete set of disclaimers and disclosures applicable to this content are available at: wallstwire.ai/disclosures. Market size figures or other estimates referenced in this article are quoted from publicly available sources; we do not independently verify or endorse them, and additional figures or estimates may exist. This article should not be considered an official communication of the issuer.





