Canada's Generic Ozempic Revolution Begins Today: Vimy Pharma Leads Charge as Patent Protections Expire
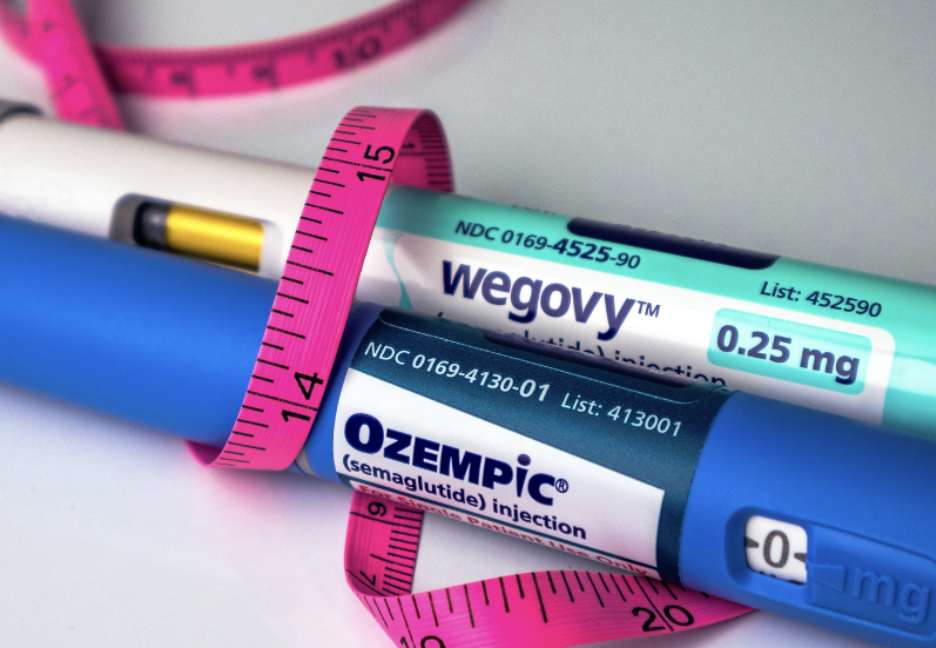
January 4, 2026, marks a watershed moment in the global GLP-1 market as Canada becomes the first country where Novo Nordisk's blockbuster diabetes and weight-loss drug Ozempic loses patent protection. Leading the charge into this newly opened market is Toronto-based Vimy Pharma, a startup founded by former Novo Nordisk executives who helped bring the original drug to Canada and now plan to challenge their former employer with a domestically manufactured generic version.
The timing couldn't be more significant. As millions of patients worldwide struggle with the high cost and limited availability of GLP-1 medications, Canada's unique patent situation offers a glimpse into the future of affordable semaglutide access. Unlike other major markets where Novo Nordisk's patents remain intact until the early 2030s, Canada presents an unprecedented opportunity for generic competition due to an unusual administrative oversight.
The Patent Lapse That Changed Everything
The story behind Canada's early generic opportunity reads like a pharmaceutical cautionary tale. In 2019, Novo Nordisk failed to pay a routine $450 CAD maintenance fee for its Canadian semaglutide compound patent, permanently forfeiting protection in what industry observers describe as an extraordinary oversight. Combined with the scheduled expiration of Ozempic's eight-year data protection today, this administrative failure has created a perfect storm for generic competition.
"We see benefits to blood glucose lowering, weight loss, of course, cardiovascular health, and they're being studied for really, a myriad of other amazing, potential indications," said Dave Suchon, Vimy Pharma's co-founder and CEO, speaking to BNN Bloomberg about the market opportunity his company aims to capture.
The irony is palpable: Suchon and co-founder Farris Smith, both former Novo Nordisk Canada executives who helped establish Ozempic's Canadian market presence, now position themselves as the drug's primary domestic challengers. Their company's name pays homage to the Battle of Vimy Ridge, reflecting a distinctly Canadian approach to taking on the Danish pharmaceutical giant.
Market Dynamics and Competitive Landscape
Vimy Pharma won't be alone in pursuing Canadian semaglutide market share. Health Canada has received nine submissions seeking approval to manufacture generic semaglutide, including applications from established players like Sandoz Canada, Apotex, Teva Canada, Taro Pharmaceuticals, and Aspen Pharmacare Canada. This competitive influx suggests the potential for dramatic price reductions, with industry experts predicting generic versions could cost as little as 35% of current brand-name pricing.
The financial stakes are substantial. Canadian retail sales of Ozempic and Wegovy could reach $3 billion annually according to Suchon, with close to 1 million Canadians already using GLP-1 drugs. As prices fall with increased competition, this patient base could expand by several million, fundamentally reshaping Canada's approach to diabetes and obesity treatment.
However, patients shouldn't expect immediate relief. Mina Tadrous, a pharmaceutical policy expert at the University of Toronto, cautions that generic semaglutide approval is unlikely until late spring or early summer. Health Canada's 180-day review timeline for generic submissions, combined with the complex nature of semaglutide evaluation, means the regulatory pathway remains lengthy despite the patent expiration.
Manufacturing Innovation and Strategic Positioning
Vimy Pharma's approach emphasizes domestic manufacturing through a partnership with Applied Pharmaceutical Innovation (API) in Edmonton, which has received $98 million in government funding. This "made in Canada" strategy addresses both supply chain resilience concerns and nationalist sentiment, while the company's partnership with Wounded Warriors Canada reinforces its domestic branding.
The manufacturing challenge shouldn't be underestimated. Generic semaglutide represents what Health Canada describes as "complex synthetic products" that require demonstration of bioequivalence to the brand-name drug despite potential manufacturing differences. This complexity explains why established generics manufacturers like Sandoz have publicly signaled their Canadian launch intentions, targeting prices up to 70% lower than current levels.
Global Implications and Future Outlook
Canada's generic semaglutide experiment carries implications far beyond its borders. As the first major market to lose patent protection, Canada serves as a real-world laboratory for GLP-1 generic competition. Success here could influence pricing strategies and market access decisions in other jurisdictions as patents eventually expire globally.
The competitive dynamics also highlight the evolving nature of pharmaceutical innovation and market access. While Novo Nordisk maintains patent protection in most major markets, the Canadian situation demonstrates how administrative oversights can create unexpected competitive opportunities, potentially accelerating patient access to life-changing medications.
For healthcare systems worldwide grappling with the cost and accessibility of GLP-1 therapies, Canada's experience may provide crucial insights into managing the transition from branded to generic competition in this critical therapeutic class. As Vimy Pharma and its competitors prepare for market entry, millions of patients await the promise of more affordable access to medications that have revolutionized diabetes and obesity treatment.
The next few months will determine whether Canada's generic semaglutide revolution delivers on its promise of expanded access and reduced costs, potentially setting a precedent for similar transitions in markets worldwide as patent protections eventually expire.